MCQs
Heat Radiation, Thermal Properties Of Matter, Dual Nature Of Matter
Total Questions : 247
| Page 4 of 25 pages
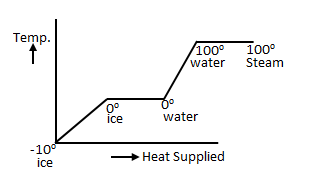
Question 31. Given that P, V and T stand for vapour pressure, volume and temperature, an ideal gas obeys the law -PV α T. If the volume is kept constant, P α T. With decreasing temperature the vapour pressure drops, until P = 0 at some temperature T0. Since P cannot be less than zero, or negative, T0 naturally becomes the lowest temperature that we can reach, and can be used as a "natural” lower fixed point while constructing a temperature scale. This is the "absolute zero”, which in the Celsius scale is - 273.150C.
To construct an absolute temperature scale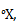 , where the absolute zero is naturally 0
, where the absolute zero is naturally 0 . Let us choose the triple point of mercury (-38.80C, at 0.2 mPa) as our upper fixed point, and give assign it a value 100
. Let us choose the triple point of mercury (-38.80C, at 0.2 mPa) as our upper fixed point, and give assign it a value 100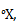 . What will be the boiling point of water on this scale?
. What will be the boiling point of water on this scale?
To construct an absolute temperature scale
Answer: Option A. -> f1−f2f2t1−f1t2
:
A
As with the rise in temperature, the liquid undergoes volume expansion therefore the fraction of solid submerged in liquid increases.
Fraction of solid submerged at t1∘C=f1 = Volume of displaced liquid =V0(1+γt1)……(i) and fraction of solid submerged at t2∘C=f2= Volume of displaced liquid =V0(1+γt2)……(ii)
From (i) and (ii) f1f2=1+γt11+γt2 ⇒ γ=f1−f2f2t1−f1t2
:
A
As with the rise in temperature, the liquid undergoes volume expansion therefore the fraction of solid submerged in liquid increases.
Fraction of solid submerged at t1∘C=f1 = Volume of displaced liquid =V0(1+γt1)……(i) and fraction of solid submerged at t2∘C=f2= Volume of displaced liquid =V0(1+γt2)……(ii)
From (i) and (ii) f1f2=1+γt11+γt2 ⇒ γ=f1−f2f2t1−f1t2
Answer: Option B. -> 1625×100
:
B
For a constant volume gas thermometer temperature in ∘centigrade is given as
Tc=P−P0P100−P0×100∘C ⇒ Tc=13−(−3)22−(−3)×100∘C=1625×100
:
B
For a constant volume gas thermometer temperature in ∘centigrade is given as
Tc=P−P0P100−P0×100∘C ⇒ Tc=13−(−3)22−(−3)×100∘C=1625×100
Answer: Option D. -> 0.135
:
D
Heat required Q=(1.1+0.02)×103×1×(80−15)=72800cal.
Therefore, mass of steam condensed (in kg)
m=QL=72800540×10−3=0.135kg.
:
D
Heat required Q=(1.1+0.02)×103×1×(80−15)=72800cal.
Therefore, mass of steam condensed (in kg)
m=QL=72800540×10−3=0.135kg.
Answer: Option B. -> l1l2=α2α1
:
B
L1=l1[1+α1ΔT]L2=l2[1+α2ΔT]L1+L2=(l1+l2)+(ΔT)(l1α1−l2α2)IfL1−L2istobeindependentofΔT,thenl1α1−l2α2=0⇒l1l2=α1α2l1l2=α2α1
:
B
L1=l1[1+α1ΔT]L2=l2[1+α2ΔT]L1+L2=(l1+l2)+(ΔT)(l1α1−l2α2)IfL1−L2istobeindependentofΔT,thenl1α1−l2α2=0⇒l1l2=α1α2l1l2=α2α1
Answer: Option C. -> Will remain almost stationary
:
C
Conceptual
:
C
Conceptual
Answer: Option A. -> 0.130
:
A
Heat lost by steam = Heat gained by water + calorimeter
∴mL+ms(100−80)=1.12×s×(80−15)
or m[540+(1×20)]=1.12×1×65
or m=1.12×1×65560=65500kgorm=0.13kg
:
A
Heat lost by steam = Heat gained by water + calorimeter
∴mL+ms(100−80)=1.12×s×(80−15)
or m[540+(1×20)]=1.12×1×65
or m=1.12×1×65560=65500kgorm=0.13kg
Question 39. Browsing through Mr. Fox's old scientific notes, Bruce Wayne encounters a secret temperature scale, which Mr. Fox called Z, in order to keep the Batmobile's technology a secret. On that scale, the freezing and the boiling points of water were recorded to be -70Z and 780Z, respectively. The records instruct to maintain the coolant temperature at -240Z for top speed. Mr. Wayne, naturally comfortable with the Fahrenheit scale due to his American upbringing, needs to calculate the coolant temperature in 0F. Help him choose the correct value.
Answer: Option A. -> 1.24
:
A
V′V = ρσ = Fraction of volume of sphere submerged = η(say)
To find % change in η, i.e., (η′η−η)×100
Or(η′η−η)×100 = [(ρσ)′(ρσ)−1]×100
= [(1+γmΔT)(1+γpΔT)]×100≃(γm−γp)ΔT×100
= (182−27)×10−6×100 = 1.24%
:
A
V′V = ρσ = Fraction of volume of sphere submerged = η(say)
To find % change in η, i.e., (η′η−η)×100
Or(η′η−η)×100 = [(ρσ)′(ρσ)−1]×100
= [(1+γmΔT)(1+γpΔT)]×100≃(γm−γp)ΔT×100
= (182−27)×10−6×100 = 1.24%