12th Grade > Chemistry
GENERAL ORGANIC CHEMISTRY MCQs
Total Questions : 23
| Page 2 of 3 pages
Answer: Option C. -> Free radical substitution
:
C
This is a good example of a photochemical reaction. A reaction brought about by light.
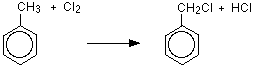
One of the hydrogen atoms in the methyl group has been replaced by a chlorine atom, so this is a substitution reaction. However, the reaction doesn't stop there, and all three hydrogens in the methyl group can, in turn, be replaced by chlorine atoms.
:
C
This is a good example of a photochemical reaction. A reaction brought about by light.
One of the hydrogen atoms in the methyl group has been replaced by a chlorine atom, so this is a substitution reaction. However, the reaction doesn't stop there, and all three hydrogens in the methyl group can, in turn, be replaced by chlorine atoms.
Answer: Option B. -> 2,3-dimethylbutane
:
B
C1H3−C3H|C2H3−C5H|C4H3−C6H3
Carbons labelled 2 & 3 are tertiary carbons, it is carbon attached with 3 other carbon atoms and rest of the carbons are primary in nature.
:
B
C1H3−C3H|C2H3−C5H|C4H3−C6H3
Carbons labelled 2 & 3 are tertiary carbons, it is carbon attached with 3 other carbon atoms and rest of the carbons are primary in nature.
Answer: Option B. -> False
:
B
Condensation Reaction?
In these Reactions, two same or different organic reactants unite to give a product withthe elimination of another simple molecule.
:
B
Condensation Reaction?
In these Reactions, two same or different organic reactants unite to give a product withthe elimination of another simple molecule.
Answer: Option B. -> I,I,II,II
:
B
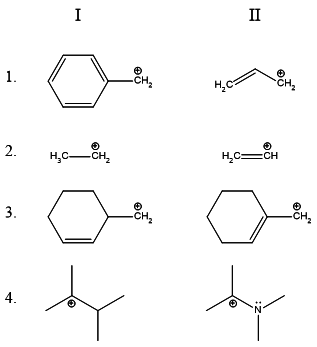
For part 1., we have to choose the more stable of benzyl and allyl
carbocations. The benzyl carbocation has more resonance contributors.
In theory, it can be considered to be more stable.
For part 2., we have to choose the more stable cation between an
1∘ alkyl and a vinyl cation. The latter is more unstable because
the positive charge rests on a carbon having a double bond- which has
more %s-character and is thus more electronegative than the alkane's
carbon bearing the positive charge. The primary alkyl carbocation is thus
more stable.
For part 3., we have a straightforward case where an allyl carbocation is
more stable than a primary alkyl carbocation.
For part 4., the lone pair on the adjacent nitrogen stabilizes the positive charge.
Hence the resonance stabilized carbocation is more stable!
:
B
For part 1., we have to choose the more stable of benzyl and allyl
carbocations. The benzyl carbocation has more resonance contributors.
In theory, it can be considered to be more stable.
For part 2., we have to choose the more stable cation between an
1∘ alkyl and a vinyl cation. The latter is more unstable because
the positive charge rests on a carbon having a double bond- which has
more %s-character and is thus more electronegative than the alkane's
carbon bearing the positive charge. The primary alkyl carbocation is thus
more stable.
For part 3., we have a straightforward case where an allyl carbocation is
more stable than a primary alkyl carbocation.
For part 4., the lone pair on the adjacent nitrogen stabilizes the positive charge.
Hence the resonance stabilized carbocation is more stable!
Answer: Option A. -> CH3 − CH2 − CH3|C|OH−CH2−CH3
:
A
CH3−CH2−CH3|C|OH−CH2−CH3H+−−→
CH3−CH2−CH3|C|⊕−CH2−CH3
Higher the stability of the carbocation, more easily it will be dehydrated.
:
A
CH3−CH2−CH3|C|OH−CH2−CH3H+−−→
CH3−CH2−CH3|C|⊕−CH2−CH3
Higher the stability of the carbocation, more easily it will be dehydrated.
Answer: Option C. -> −NR2
:
C
Relative inductive effects have been experimentally measured with reference to hydrogen, in decreasing order of -I effect or increasing order of +I effect, as follows:
–NH3+> –NO2> –SO2R > –CN > –SO3H > –CHO > –CO > –COOH > –COCl> –CONH2>
–F > –Cl > –Br > –I > –OR > -OH > –NH2> –C6H5> –CH=CH2> –H
From this, you can get the required answer.
:
C
Relative inductive effects have been experimentally measured with reference to hydrogen, in decreasing order of -I effect or increasing order of +I effect, as follows:
–NH3+> –NO2> –SO2R > –CN > –SO3H > –CHO > –CO > –COOH > –COCl> –CONH2>
–F > –Cl > –Br > –I > –OR > -OH > –NH2> –C6H5> –CH=CH2> –H
From this, you can get the required answer.
Answer: Option C. -> four
:
C
Nature of bondNumber of sigma bondsNumber of pi-bondsSingle bond10Double bond11Triple bond12In the compound CH2=CH−CH=CH−C≡CH, there are (2×1) + (1×2) = 4 pi-bonds in total.
:
C
Nature of bondNumber of sigma bondsNumber of pi-bondsSingle bond10Double bond11Triple bond12In the compound CH2=CH−CH=CH−C≡CH, there are (2×1) + (1×2) = 4 pi-bonds in total.
Answer: Option A. -> sp2 and sp2
:
A
sp and sp2
N≡spC1−sp2CH2=C3H2
:
A
sp and sp2
N≡spC1−sp2CH2=C3H2
Answer: Option D. -> NO+2
:
D
The process of nitration takes place as below
HONO2+2H2SO4⇌ H3O++2HSO−4+NO+2
(nitronium ion)
The electrophile responsible for nitration is
NO+2 ion.
:
D
The process of nitration takes place as below
HONO2+2H2SO4⇌ H3O++2HSO−4+NO+2
(nitronium ion)
The electrophile responsible for nitration is
NO+2 ion.
Answer: Option B. -> tetrahedral
:
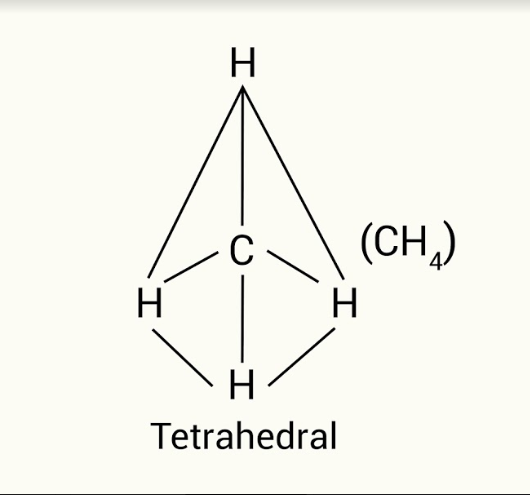
B
Carbon has four electrons in its valence shell. In methane molecule, the carbon atom forms covalent bonds with four different hydrogen atoms. There are no lone pairs on carbon atom. Therefore, the four hydrogen atoms are arranged at four corners of tetrahedron and carbon at the centre of the tetrahedron. The structure of methane molecule is tetrahedral.
:
B
Carbon has four electrons in its valence shell. In methane molecule, the carbon atom forms covalent bonds with four different hydrogen atoms. There are no lone pairs on carbon atom. Therefore, the four hydrogen atoms are arranged at four corners of tetrahedron and carbon at the centre of the tetrahedron. The structure of methane molecule is tetrahedral.