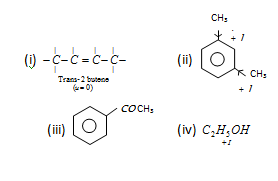
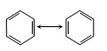
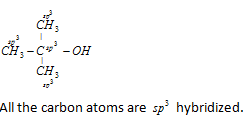12th Grade > Chemistry
GENERAL ORGANIC CHEMISTRY MCQs
Total Questions : 23
| Page 1 of 3 pages
Answer: Option A. ->
:
B
Tertiary carbocation is most stable among primary, secondary and tertiary due to more hyper conjugation.
:
B
Tertiary carbocation is most stable among primary, secondary and tertiary due to more hyper conjugation.
Answer: Option A. ->
:
D
In p-nitrophenol, NO2group is an electron withdrawing group but in structure (d) it is shown as electron releasing group.
:
D
In p-nitrophenol, NO2group is an electron withdrawing group but in structure (d) it is shown as electron releasing group.
Answer: Option A. ->
:
B
All free radicals have planar geometry but bicyclo compounds free radical has a pyramidal shape.
Because of steric strain, the carbon atom carrying the unpaired electron cannot assume a planar geometry.
:
B
All free radicals have planar geometry but bicyclo compounds free radical has a pyramidal shape.
Because of steric strain, the carbon atom carrying the unpaired electron cannot assume a planar geometry.
Answer: Option C. -> Sp2 in C-3 and C-5 and SP in C-4
:
C
Carbon which has 3σ bonds and 1π bond in C-3 and C-5 sp2 hybridised.
Carbon which has 3σ bonds and 1π bond as in C-4 is sp hybridised.
:
C
Carbon which has 3σ bonds and 1π bond in C-3 and C-5 sp2 hybridised.
Carbon which has 3σ bonds and 1π bond as in C-4 is sp hybridised.
Answer: Option B. -> sp2
:
B
Look at the structure of benzene.
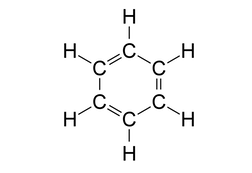
Every carbon atom in the benzene ring has a double bond with a neighbouring carbon atom and a single bond with another carbon atom. It is also bonded to one hydrogen atom with a single bond.
So, each carbon atom has 3 sigma bonds and 1 pi bond. This is possible only if the carbon atom issp2 hybridized where we get 3 sp2 hybrid orbitals to form 3 sigma bonds and 1 unhybridised p orbital to form a pi bond.
Another feature of sp2 hybridisation is that it leads to a trigonal planar geometry which is also true in this case.
:
B
Look at the structure of benzene.

Every carbon atom in the benzene ring has a double bond with a neighbouring carbon atom and a single bond with another carbon atom. It is also bonded to one hydrogen atom with a single bond.
So, each carbon atom has 3 sigma bonds and 1 pi bond. This is possible only if the carbon atom issp2 hybridized where we get 3 sp2 hybrid orbitals to form 3 sigma bonds and 1 unhybridised p orbital to form a pi bond.
Another feature of sp2 hybridisation is that it leads to a trigonal planar geometry which is also true in this case.
Answer: Option C. -> Electrophilic substitution reaction
:
C
It so happens that the most common type of reaction in aromatic compounds is an electrophilic substitution reaction.
This is because the benzene molecule is electron-rich.
:
C
It so happens that the most common type of reaction in aromatic compounds is an electrophilic substitution reaction.
This is because the benzene molecule is electron-rich.