8th Grade > Chemistry
COMBUSTION AND FLAME MCQs
Total Questions : 56
| Page 2 of 6 pages
Answer: Option B. -> Chemical process
:
B
Combustion refers to the process where a substance burns in the presence of oxygen, giving off heat and light in the process.
The way to recognize a chemical change is to check whether a new substance is being created or not. During combustion, the fuel reacts with oxygen to form some new gases. So, a new substance is definitely formed. So, combustion is a chemical change.
:
B
Combustion refers to the process where a substance burns in the presence of oxygen, giving off heat and light in the process.
The way to recognize a chemical change is to check whether a new substance is being created or not. During combustion, the fuel reacts with oxygen to form some new gases. So, a new substance is definitely formed. So, combustion is a chemical change.
Answer: Option A. -> True
:
A
White phosphorusis highly reactive and has a very low ignition temperature. It spontaneouslyignites at about 30 °C in air. So if the room temperature is more than30 °C, then it would catch fire.
It burns with awhiteflame producing clouds ofwhitesmoke.It is usually stored under water to prevent exposure to air.
:
A
White phosphorusis highly reactive and has a very low ignition temperature. It spontaneouslyignites at about 30 °C in air. So if the room temperature is more than30 °C, then it would catch fire.
It burns with awhiteflame producing clouds ofwhitesmoke.It is usually stored under water to prevent exposure to air.
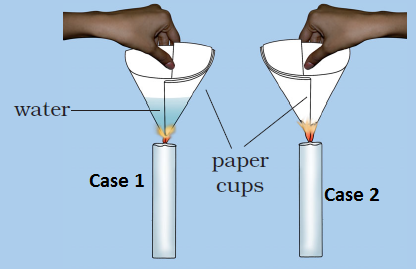
Answer: Option B. -> Case 2
:
B
In case 2, the empty paper cup reaches its ignition temperature and catches fire. In case 1, the heat supplied to the paper cup is transferred to water in it. So, in the presence of water, the ignition temperature of the paper is not reached. So, the paper would not burn in case 1.
:
B
In case 2, the empty paper cup reaches its ignition temperature and catches fire. In case 1, the heat supplied to the paper cup is transferred to water in it. So, in the presence of water, the ignition temperature of the paper is not reached. So, the paper would not burn in case 1.
Answer: Option A. -> True
:
A
A certain minimum temperature is required to initiate combustion reaction. This temperature is called ignition temperature.
A type of combustion where an external agent is not required and the material catches fire automatically is called spontaneous combustion.When the ignition temperature is lesser than the room temperature, combustion takes places on its own without the application of any external agent.
:
A
A certain minimum temperature is required to initiate combustion reaction. This temperature is called ignition temperature.
A type of combustion where an external agent is not required and the material catches fire automatically is called spontaneous combustion.When the ignition temperature is lesser than the room temperature, combustion takes places on its own without the application of any external agent.
Answer: Option A. -> True
:
A
The calorific value of a fuel is expressed in a unit called kilojoule per kg (kJ/kg). Calorific value is the term that is used to measure the efficiency of a fuel.
:
A
The calorific value of a fuel is expressed in a unit called kilojoule per kg (kJ/kg). Calorific value is the term that is used to measure the efficiency of a fuel.
Answer: Option A. -> Explosion
:
A
When a cracker is ignited, a sudden reaction takes place with the evolution of heat, light and sound. A large amount of gas liberated during this reaction. Such a reaction is called explosion.
:
A
When a cracker is ignited, a sudden reaction takes place with the evolution of heat, light and sound. A large amount of gas liberated during this reaction. Such a reaction is called explosion.
Answer: Option A. -> True
:
A
If air is blown around coal, then air circulation increases the supply of oxygen gas which will help in the complete combustion of coal. Supply of sufficient amount of oxygen gas lets the coal burn completely.
:
A
If air is blown around coal, then air circulation increases the supply of oxygen gas which will help in the complete combustion of coal. Supply of sufficient amount of oxygen gas lets the coal burn completely.
Answer: Option C. -> heat and oxygen
:
C
Water removes heat and cuts off the supply of oxygen when it is used as a fire extinguisher.
Water cools the combustible material so that its temperature is brought below its ignition temperature. This prevents the fire from spreading. Water vapours also surround the combustible material, helping in cutting off the supply of air so that the fire is exhausted. Butwater cannot be used for fires involving electrical equipment and inflammable substances.
:
C
Water removes heat and cuts off the supply of oxygen when it is used as a fire extinguisher.
Water cools the combustible material so that its temperature is brought below its ignition temperature. This prevents the fire from spreading. Water vapours also surround the combustible material, helping in cutting off the supply of air so that the fire is exhausted. Butwater cannot be used for fires involving electrical equipment and inflammable substances.
Answer: Option C. -> The blanket cuts off the supply of oxygen which in turn stops combustion.
:
C
The essential requirements for producing fire are fuel, air(to supply oxygen),and heat. Fire can be controlled by removing one or more of these requirements.
As oxygen gas is essential for combustion, we can stop the fire by cutting the supply of oxygen. This can be done by wrapping a blanket around the person. So, it stops the combustion and the person can be saved.
:
C
The essential requirements for producing fire are fuel, air(to supply oxygen),and heat. Fire can be controlled by removing one or more of these requirements.
As oxygen gas is essential for combustion, we can stop the fire by cutting the supply of oxygen. This can be done by wrapping a blanket around the person. So, it stops the combustion and the person can be saved.