MCQs
Total Questions : 136
| Page 3 of 14 pages
Answer: Option B. -> False
Answer: (b).False
Answer: (b).False
Answer: Option B. -> Phosphorous
Answer: (b).Phosphorous
Answer: (b).Phosphorous
Answer: Option B. -> Thermionic emission detector
Answer: (b).Thermionic emission detector
Answer: (b).Thermionic emission detector
Answer: Option A. -> Argon ionisation detector
Answer: (a).Argon ionisation detector
Answer: (a).Argon ionisation detector
Answer: Option C. -> Graphite
Answer: (c).Graphite
Answer: (c).Graphite
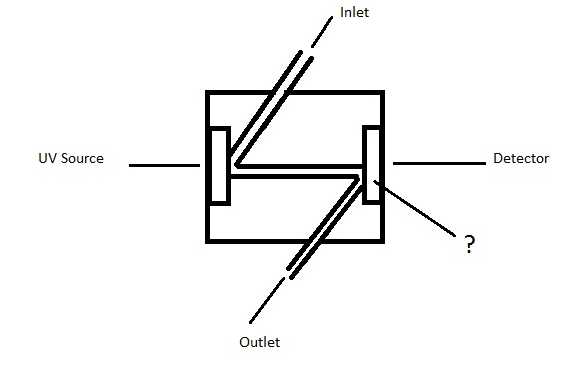
Answer: Option D. -> Quartz window
Answer: (d).Quartz window
Answer: (d).Quartz window
Answer: Option A. -> True
Answer: (a).True
Answer: (a).True
Answer: Option B. -> Adsorption of gaseous substances on solid surface
Answer: (b).Adsorption of gaseous substances on solid surface
Answer: (b).Adsorption of gaseous substances on solid surface
Answer: Option C. -> Leads to semi-permanent retention of the analyte
Answer: (c).Leads to semi-permanent retention of the analyte
Answer: (c).Leads to semi-permanent retention of the analyte
Answer: Option B. -> Hydrogen sulphide
Answer: (b).Hydrogen sulphide
Answer: (b).Hydrogen sulphide