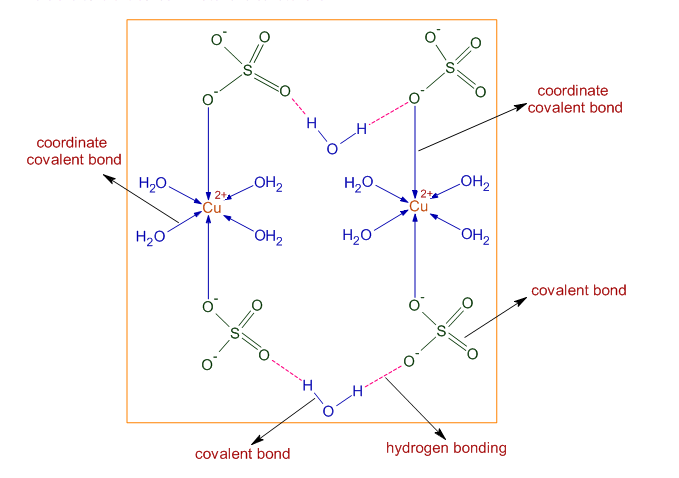
12th Grade > Chemistry
CHEMICAL BONDING MCQs
Total Questions : 30
| Page 2 of 3 pages
Answer: Option B. -> CH3F, CH4
:
B
F is not linked to directly to hydrogen
:
B
F is not linked to directly to hydrogen
Answer: Option B. -> H2O> HF> NH3> CH4
:
B
H2O has higher b.pt among hydrogen bonded molecules
:
B
H2O has higher b.pt among hydrogen bonded molecules
Answer: Option D. -> A is false but R is true
:
D
Due to stronger hydrogen bonding in water, it has a higher boilind point.
:
D
Due to stronger hydrogen bonding in water, it has a higher boilind point.
Answer: Option B. -> sp3, dsp2, sp3d , sp2
:
B
Pt would form an inner orbital d complex and P would form an outer orbital d complex.
This eliminates all the other options and you have b as the correct option.
:
B
Pt would form an inner orbital d complex and P would form an outer orbital d complex.
This eliminates all the other options and you have b as the correct option.
Answer: Option C. -> I−>Br−>Cl−>F−
:
C
The polarisability of an anion also depends on its size and charge – the larger the negative ion and the larger its charge the more polarisable it becomes, that is, more is the size of anion more will be the polarisability.
:
C
The polarisability of an anion also depends on its size and charge – the larger the negative ion and the larger its charge the more polarisable it becomes, that is, more is the size of anion more will be the polarisability.
Answer: Option B. -> The extent of H-bonding decreases from water to methanol while it is absent in ether
:
B
Water has strongest hydrogen bond of the three
:
B
Water has strongest hydrogen bond of the three
Answer: Option B. -> C in C2H4
:
B
SO2 & C2H4 have sp2 hybridisation.
The Carbon atoms in C2H2 and CO2 has sphybridisation.
The Carbon atom in CH4 hassp3hybridisation.
:
B
SO2 & C2H4 have sp2 hybridisation.
The Carbon atoms in C2H2 and CO2 has sphybridisation.
The Carbon atom in CH4 hassp3hybridisation.
Answer: Option D. -> II > I = III > IV
:
D
Para compound has zero dipole moment and ortho compound has maximum value.
If we calculate the vector addition, magnitudes of dipole moment for I and III turn
out to be the same.
:
D
Para compound has zero dipole moment and ortho compound has maximum value.
If we calculate the vector addition, magnitudes of dipole moment for I and III turn
out to be the same.
Answer: Option C. -> 1160
:
C
This is HF in its solid form, it has a bond angle of 116 degrees.
:
C
This is HF in its solid form, it has a bond angle of 116 degrees.