Question
Look at the following reaction:
LiH+AlH3⟶LiAlH4
Here AlH3 and LiH act as:
LiH+AlH3⟶LiAlH4
Here AlH3 and LiH act as:
Answer: Option A
:
A
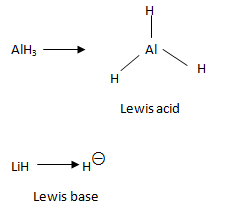
As shown in the figure, the central metal atom in AlH3 has only six valence electrons. Hence, it could accept a pair of electrons (Lewis acid) to complete its octet. On the other hand, H− is a Lewis base because it has two valence electrons thus having a completely filled shell. Further, since the ion readily shares or donates electrons - it acts as a Lewis base.
Was this answer helpful ?
:
A

As shown in the figure, the central metal atom in AlH3 has only six valence electrons. Hence, it could accept a pair of electrons (Lewis acid) to complete its octet. On the other hand, H− is a Lewis base because it has two valence electrons thus having a completely filled shell. Further, since the ion readily shares or donates electrons - it acts as a Lewis base.
Was this answer helpful ?


















Submit Solution