Question
Each carbon atom in benzene is in which state of hybridization?
Answer: Option B
:
B
Look at the structure of benzene.
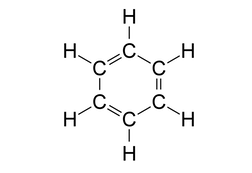
Every carbon atom in the benzene ring has a double bond with a neighbouring carbon atom and a single bond with another carbon atom. It is also bonded to one hydrogen atom with a single bond.
So, each carbon atom has 3 sigma bonds and 1 pi bond. This is possible only if the carbon atom issp2 hybridized where we get 3 sp2 hybrid orbitals to form 3 sigma bonds and 1 unhybridised p orbital to form a pi bond.
Another feature of sp2 hybridisation is that it leads to a trigonal planar geometry which is also true in this case.
Was this answer helpful ?
:
B
Look at the structure of benzene.

Every carbon atom in the benzene ring has a double bond with a neighbouring carbon atom and a single bond with another carbon atom. It is also bonded to one hydrogen atom with a single bond.
So, each carbon atom has 3 sigma bonds and 1 pi bond. This is possible only if the carbon atom issp2 hybridized where we get 3 sp2 hybrid orbitals to form 3 sigma bonds and 1 unhybridised p orbital to form a pi bond.
Another feature of sp2 hybridisation is that it leads to a trigonal planar geometry which is also true in this case.
Was this answer helpful ?


















Submit Solution