Question
At low temperature, say below (−11∘C) nitrogen dioxide exist as a trimer.
Answer: Option B
:
B
At low temperatures like (−11∘C) nitrogen dioxide exist as a dimer, not trimer. This is an observation.
2NO2⇌N2O4
(Brown gas) (Colorless solid)
N2O4 is a diamagnetic, colourless substance. The equilibrium shown above becomes more and more favoured in the forward direction as we lower the temperature. Above (21∘C), NO2 is a reddish-brown gas and below (21∘C), NO2 is a yellowish-brown liquid. On cooling below (−9∘C) it dimerizes into the colourless N2O4 in the . For the dimer, in the liquid and gaseous states, the following structure is proposed.
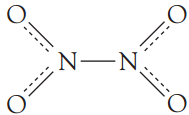
The dimer formation can be attributed to the overlap of the sp2 hybrid orbitals containing the “odd” electrons. The consequent N—N bonding molecular orbital exhibits weak bonding character. In fact,
the N—N bond energy is only about 60 kJ.mol−1.
Was this answer helpful ?
:
B
At low temperatures like (−11∘C) nitrogen dioxide exist as a dimer, not trimer. This is an observation.
2NO2⇌N2O4
(Brown gas) (Colorless solid)
N2O4 is a diamagnetic, colourless substance. The equilibrium shown above becomes more and more favoured in the forward direction as we lower the temperature. Above (21∘C), NO2 is a reddish-brown gas and below (21∘C), NO2 is a yellowish-brown liquid. On cooling below (−9∘C) it dimerizes into the colourless N2O4 in the . For the dimer, in the liquid and gaseous states, the following structure is proposed.

The dimer formation can be attributed to the overlap of the sp2 hybrid orbitals containing the “odd” electrons. The consequent N—N bonding molecular orbital exhibits weak bonding character. In fact,
the N—N bond energy is only about 60 kJ.mol−1.
Was this answer helpful ?


















Submit Solution