12th Grade > Chemistry
P-BLOCK GROUP 15 - PNICTOGENS MCQs
Total Questions : 14
| Page 1 of 2 pages
Answer: Option D. -> Heating NH4NO2 carefully
:
D
HeatingNH4NO3 carefully produces N2O but heatingNH4NO2 liberates N2 gas.
:
D
HeatingNH4NO3 carefully produces N2O but heatingNH4NO2 liberates N2 gas.
Answer: Option A. -> True
:
A
Nitrogen forms a number of oxides:
N2O is nitrous oxide, NO is nitric oxide,N2O3 is dinitrogen trioxide, NO2 is nitrogen dioxide and N2O5 is dinitrogen pentoxide. Between Nitrogen and Oxygen, which is more electronegative? It is oxygen.
Let us briefly recall the rules for allocating oxidation numbers:
Let the oxidation state of the central Nitrogen atom be ‘x’. Let us apply the usual rules from above:
Hydrogen has an oxidation number of +1 while oxygen has -2. Also, the sum of oxidation states of all the atoms in a neutral atom is 0.
Hence,
2x + (-2) = 0;
Solving, x = +1
Now try that for the remaining oxides. You will see that Nitrogen is in its +5 oxidation state in N2O5.
:
A
Nitrogen forms a number of oxides:
N2O is nitrous oxide, NO is nitric oxide,N2O3 is dinitrogen trioxide, NO2 is nitrogen dioxide and N2O5 is dinitrogen pentoxide. Between Nitrogen and Oxygen, which is more electronegative? It is oxygen.
Let us briefly recall the rules for allocating oxidation numbers:
- The oxidation number of an element in its free state is zero — for example, H2, S8,P4 etc.
- In the case monoatomic ions, it is the same as the charge on the ion, for example: Ca2+=+2 and N3−=−3
- For a neutral compound, the sum of all oxidation numbers is zero. The sum of all oxidation numbers in apolyatomic(many-atom) ion is equal to the overall charge on the ion.
- The first two groups - alkali metal (IA family) and alkaline earth metal (IIA family) generally tend to have +1 and +2 oxidation states respectively.
- For most compounds, the oxidation number of oxygen is generally –2. There is, however, an exception in the case of peroxides(H2O2, Na2O2). Usually, in cases where an - O – O bond is involved, oxygen attains an oxidation number of –1. If the oxygen is bonded to fluorine, the number is +1.
- Fluorine is the most electronegative element in the periodic table. It almost always has the oxidation number as –1. Chlorine, bromine, and iodine usually have an oxidation number of –1, unless they’re in combination with an oxygen, fluorine or between themselves (halogens).
- Usually, the oxidation state of hydrogen in a compound is +1. As an exception they tend to have –1 oxidation states in binary metal hydrides like NaH LiH etc.
Let the oxidation state of the central Nitrogen atom be ‘x’. Let us apply the usual rules from above:
Hydrogen has an oxidation number of +1 while oxygen has -2. Also, the sum of oxidation states of all the atoms in a neutral atom is 0.
Hence,
2x + (-2) = 0;
Solving, x = +1
Now try that for the remaining oxides. You will see that Nitrogen is in its +5 oxidation state in N2O5.
Answer: Option A. -> True
:
A
Nitrous oxide produces hysterical laughter when inhaled in a moderate quantity. Hence nitrous oxide is also called “Laughing Gas”.
:
A
Nitrous oxide produces hysterical laughter when inhaled in a moderate quantity. Hence nitrous oxide is also called “Laughing Gas”.
Answer: Option B. -> False
:
B
Sulphuric acid reacts with ammonia to form ammonium sulphate
H2SO4+2NH3→(NH4)2SO4
Therefore, CaO is used to dry ammonia gas
CaO+H2O→Ca(OH)2
:
B
Sulphuric acid reacts with ammonia to form ammonium sulphate
H2SO4+2NH3→(NH4)2SO4
Therefore, CaO is used to dry ammonia gas
CaO+H2O→Ca(OH)2
Answer: Option A. -> True
:
A
Cyanamide is an important organic chemical, which is used in agriculture and in the production of various pharmaceuticals and other organic compounds. It can be thought of as a nitrile group attached to an amine group.
Calcium Cyanamide is CaNCN, an inorganic compound that is extensively used as a fertilizer. It is synthesized by the famous Frank-Caro process (also known as the cyanamide process) where Calcium Carbide is heated with N2gas in a reactor at 1273 K. The reaction is exothermic:
CaC2 + N2 → CaNCN + C
( Calcium (Nitrogen) ( Calcium (Carbon)
Carbide) Cyanamide)
CaCN2 + 4H2O → Ca(OH)2 + CO2 + 2NH3
(Calcium hydroxide) (Ammonia)
:
A
Cyanamide is an important organic chemical, which is used in agriculture and in the production of various pharmaceuticals and other organic compounds. It can be thought of as a nitrile group attached to an amine group.
Calcium Cyanamide is CaNCN, an inorganic compound that is extensively used as a fertilizer. It is synthesized by the famous Frank-Caro process (also known as the cyanamide process) where Calcium Carbide is heated with N2gas in a reactor at 1273 K. The reaction is exothermic:
CaC2 + N2 → CaNCN + C
( Calcium (Nitrogen) ( Calcium (Carbon)
Carbide) Cyanamide)
CaCN2 + 4H2O → Ca(OH)2 + CO2 + 2NH3
(Calcium hydroxide) (Ammonia)
:
With Thionyl chloride, P4givesPCl3
P4+8SOCl2→4PCl3+3S2Cl2+4SO2
While reacting with Sulphuryl Chloride, P4 yields Phosphorus Pentachloride:
P4+10SO2Cl2→4PCl5+10SO2
Answer: Option B. -> False
:
B
At low temperatures like (−11∘C) nitrogen dioxide exist as a dimer, not trimer. This is an observation.
2NO2⇌N2O4
(Brown gas) (Colorless solid)
N2O4 is a diamagnetic, colourless substance. The equilibrium shown above becomes more and more favoured in the forward direction as we lower the temperature. Above (21∘C), NO2 is a reddish-brown gas and below (21∘C), NO2 is a yellowish-brown liquid. On cooling below (−9∘C) it dimerizes into the colourless N2O4 in the . For the dimer, in the liquid and gaseous states, the following structure is proposed.
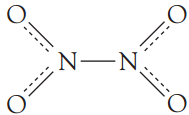
The dimer formation can be attributed to the overlap of the sp2 hybrid orbitals containing the “odd” electrons. The consequent N—N bonding molecular orbital exhibits weak bonding character. In fact,
the N—N bond energy is only about 60 kJ.mol−1.
:
B
At low temperatures like (−11∘C) nitrogen dioxide exist as a dimer, not trimer. This is an observation.
2NO2⇌N2O4
(Brown gas) (Colorless solid)
N2O4 is a diamagnetic, colourless substance. The equilibrium shown above becomes more and more favoured in the forward direction as we lower the temperature. Above (21∘C), NO2 is a reddish-brown gas and below (21∘C), NO2 is a yellowish-brown liquid. On cooling below (−9∘C) it dimerizes into the colourless N2O4 in the . For the dimer, in the liquid and gaseous states, the following structure is proposed.

The dimer formation can be attributed to the overlap of the sp2 hybrid orbitals containing the “odd” electrons. The consequent N—N bonding molecular orbital exhibits weak bonding character. In fact,
the N—N bond energy is only about 60 kJ.mol−1.
Answer: Option A. -> True
:
A
HNO2(Nitrous acid) is monobasic acid because it can donate only a single proton.
Basicity is the number of protons that an acid can give. From the structure,
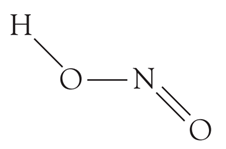
We can see that there is only one H atom and so only one proton can be abstracted from a HNO2molecule. What can you say about the basicity of nitric acid?
:
A
HNO2(Nitrous acid) is monobasic acid because it can donate only a single proton.
Basicity is the number of protons that an acid can give. From the structure,

We can see that there is only one H atom and so only one proton can be abstracted from a HNO2molecule. What can you say about the basicity of nitric acid?
Answer: Option D. -> Metal Nitrite
:
D
Nitrates on heating with lead decompose to give the corresponding nitrites and Lead (II) Oxide
NaNO3+Pb→NaNO2+PbO
:
D
Nitrates on heating with lead decompose to give the corresponding nitrites and Lead (II) Oxide
NaNO3+Pb→NaNO2+PbO
:
Let us take a look at the structure of H3PO4:
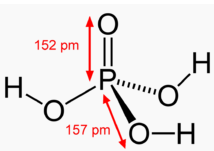
As we can see from the above structure, the Oxygen atom shown to have double bond character acts as a site to accept Hydrogen bonds while the other Hydrogen atoms attached via a single bond to Oxygen atoms can participate in Hydrogen bond. So in all, for every molecule there are 3 donor sites and one acceptor site.
















