12th Grade > Chemistry
P-BLOCK GROUP 18 - NOBLE GASES MCQs
Total Questions : 12
| Page 2 of 2 pages
Answer: Option D. -> Linear and trigonal bipyramidal
:
D
Let us look at the ground state valence electronic configuration of Xe and the first excited state:
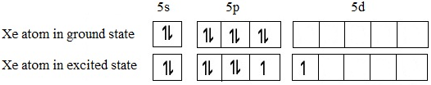
So there are 3 lone pairs of electrons and two bond pairs (each unpaired electron shown above will pair with exactly one electron from either of the Fluorine atoms). These orbitals may be assumed to be hybridized correspond to sp3d geometry.
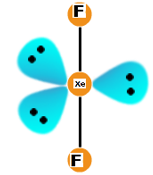
The lone pairs occupy three ends of a symmetrical plane while the bond pairs will be axial or perpendicular to the plane containing the 3 electron pairs.
Shape is linear (due to perfect geometry) while the geometry is trigonal bipyramidal. What will be the F – Xe – F bond angle? Yes. It will will be 180 degrees.
The geometry will be like:
:
D
Let us look at the ground state valence electronic configuration of Xe and the first excited state:

So there are 3 lone pairs of electrons and two bond pairs (each unpaired electron shown above will pair with exactly one electron from either of the Fluorine atoms). These orbitals may be assumed to be hybridized correspond to sp3d geometry.
The lone pairs occupy three ends of a symmetrical plane while the bond pairs will be axial or perpendicular to the plane containing the 3 electron pairs.
Shape is linear (due to perfect geometry) while the geometry is trigonal bipyramidal. What will be the F – Xe – F bond angle? Yes. It will will be 180 degrees.
The geometry will be like:

Answer: Option B. -> False
:
B
Xenon Fluorides are stored in Nickel or Monel containers. They cannot be stored in glass containers or quartz containers as they react with SiO2 as:
2XeF6(s)+SiO2(s)→SiF4(s)+2XeOF4(s)
2XeOF4(s)+SiO2(s)→SiF4(s)+2XeO2F2(s)
2XeO2F2(s)+SiO2(s)→SiF4(s)+2XeO3(s)
Also, the first two reactions may be used to prepare Xenon oxyfluorides. Further, Xenon oxyfluorides can also be prepared by the controlled, partial hydrolysis of Xenon fluorides.
Hydrolysis of XeF6is a multi-step process. Initially Xenon oxide tetrafluoride – XeOF4is formed.
XeF6(s)+H2O(l)→XeOF4(l)+2HF(l)
Followed by
XeOF4(l)+2H2O(l)→XeO3(s)+4HF(l)
:
B
Xenon Fluorides are stored in Nickel or Monel containers. They cannot be stored in glass containers or quartz containers as they react with SiO2 as:
2XeF6(s)+SiO2(s)→SiF4(s)+2XeOF4(s)
2XeOF4(s)+SiO2(s)→SiF4(s)+2XeO2F2(s)
2XeO2F2(s)+SiO2(s)→SiF4(s)+2XeO3(s)
Also, the first two reactions may be used to prepare Xenon oxyfluorides. Further, Xenon oxyfluorides can also be prepared by the controlled, partial hydrolysis of Xenon fluorides.
Hydrolysis of XeF6is a multi-step process. Initially Xenon oxide tetrafluoride – XeOF4is formed.
XeF6(s)+H2O(l)→XeOF4(l)+2HF(l)
Followed by
XeOF4(l)+2H2O(l)→XeO3(s)+4HF(l)
















