MCQs
Total Questions : 129
| Page 13 of 13 pages
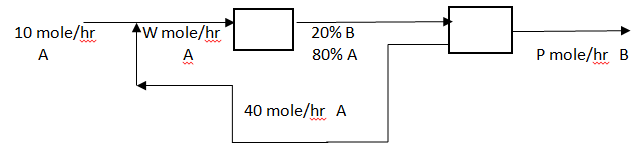
Answer: Option B. -> 20%
Answer: (b).20%
Answer: (b).20%
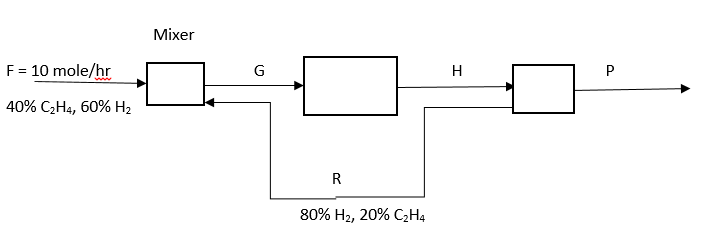
Answer: Option A. -> 10%
Answer: (a).10%
Answer: (a).10%
Answer: Option B. -> 3.6
Answer: (b).3.6
Answer: (b).3.6
Answer: Option A. -> 3.6
Answer: (a).3.6
Answer: (a).3.6
Answer: Option C. -> 4
Answer: (c).4
Answer: (c).4
Answer: Option A. -> 150 K
Answer: (a).150 K
Answer: (a).150 K
Answer: Option B. -> 8
Answer: (b).8
Answer: (b).8
Answer: Option B. -> 6 atm
Answer: (b).6 atm
Answer: (b).6 atm
Answer: Option A. -> 2
Answer: (a).2
Answer: (a).2