MCQs
Heat Radiation, Thermal Properties Of Matter, Dual Nature Of Matter
Total Questions : 247
| Page 5 of 25 pages
Question 41. It was a bright and breezy day in New York, the air was a cool 20 degrees Celsius, when Tony Stark, a.k.a the Iron Man's day took a dramatic turn as he got news of Mandarin's attack in the windy city of Chicago, and decided to immediately fly over there, in his iron body-armor, whose cavity had enough space to fit a man of 4,984 cm3 in volume, but not more.
Being a smart man, he did a quick check of two important things - his own body volume, which he found was currently 4.980 cm3, and the temperature in Chicago, which was a cold 8 degrees Celsius that day. Knowing that the coefficient of volume expansion for iron, γ, is 33.3 × 10−6/∘C, he decided to go. Check if that was a smart decision, by finding out whether the new volume of the iron suit when he reaches Chicago will crush him or not.
Being a smart man, he did a quick check of two important things - his own body volume, which he found was currently 4.980 cm3, and the temperature in Chicago, which was a cold 8 degrees Celsius that day. Knowing that the coefficient of volume expansion for iron, γ, is 33.3 × 10−6/∘C, he decided to go. Check if that was a smart decision, by finding out whether the new volume of the iron suit when he reaches Chicago will crush him or not.
Answer: Option A. -> 4.982 cm3 (he is going to be alright)
:
A
Mr. Stark's suit is made of iron, which should contract upon cooling with a coefficient γ = 33.3×10−6/∘C. As he flies from New York to Chicago, there occurs a temperature drop of 12∘C.
∴ΔT = TChicago - TNY = (8∘C- 20∘C) = −12∘C
The volume, VNY, of the suit's cavity in New York = 4,984cm3.
∴ The volume of the cavity in Chicago will be -
VChicago = VNY(1+γΔT)
= 4.984[1+33.3×10−6×(−12)]cm3
≈ 4.982cm3.
Awesome! The suit will still fit Tony Stark comfortably in Chicago, so he can just concentrate on Mandarin's onslaught. I feel much less worried about Chicago now!
:
A
Mr. Stark's suit is made of iron, which should contract upon cooling with a coefficient γ = 33.3×10−6/∘C. As he flies from New York to Chicago, there occurs a temperature drop of 12∘C.
∴ΔT = TChicago - TNY = (8∘C- 20∘C) = −12∘C
The volume, VNY, of the suit's cavity in New York = 4,984cm3.
∴ The volume of the cavity in Chicago will be -
VChicago = VNY(1+γΔT)
= 4.984[1+33.3×10−6×(−12)]cm3
≈ 4.982cm3.
Awesome! The suit will still fit Tony Stark comfortably in Chicago, so he can just concentrate on Mandarin's onslaught. I feel much less worried about Chicago now!
Answer: Option A. -> 4.982 cm3 (he is going to be alright)
:
B
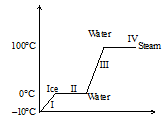 The change of ice at −10∘C into steam at 100∘C occurs in four stages.
The change of ice at −10∘C into steam at 100∘C occurs in four stages.
:
B
 The change of ice at −10∘C into steam at 100∘C occurs in four stages.
The change of ice at −10∘C into steam at 100∘C occurs in four stages.- The temperature of ice changes from −10∘Cto0∘C.
- Ice at 0∘C changes to water at 0∘C. The state changes as heat is supplied.
- Water at 0∘C changes into water at 100∘C.
- Water at 100∘C change into steam at 100∘C.The state changes as heat is supplied.
Question 43. Column-I gives some devices and Column-II gives some processes on which the functioning of these devices depend. Match the devices in Column-I with the processes in Column-II and indicates your answer by darkening appropriate bubbles in the 4 × 4 matrix given in the ORS.
Column-I
Column-II
(p) Bimetallic strip
(a) Radiation from a hot body
(q) Steam engine
(b) Energy conversion
(r) Incandescent lamp
(c) Melting
(s) Electric fuse
(d) Thermal expansion of solids
(IIT JEE 2007)
Column-I
Column-II
(p) Bimetallic strip
(a) Radiation from a hot body
(q) Steam engine
(b) Energy conversion
(r) Incandescent lamp
(c) Melting
(s) Electric fuse
(d) Thermal expansion of solids
(IIT JEE 2007)
Answer: Option A. -> (p → d);(q → b);(r → a,b);(s → b,c)
:
A
Bimetallic strip is based on thermal expansion of materials.
In stream engine, internal energy of fuel (say coal) is converted into mechanical work.
In incandescent lamp and fuse also energy is converted from electrical to heat.
Fuse is based on melting of fuse wire if suddenly current increases.
:
A
Bimetallic strip is based on thermal expansion of materials.
In stream engine, internal energy of fuel (say coal) is converted into mechanical work.
In incandescent lamp and fuse also energy is converted from electrical to heat.
Fuse is based on melting of fuse wire if suddenly current increases.
Question 44. A lead bullet strikes against a steel armor plate with the velocity of 300 meter/second. If the bullet, assuming that the impact is perfectly inelastic find the rise in temperature of the bullet. Assume that the heat produced is shared equally between the bullet and the target, specific heat of lead = 0.03 cal/g/∘C
Answer: Option A. -> 178.6∘C
:
A
Suppose m be the mass of the bullet and T be the rise in temperature.
Heat produced in the bullet
H=m×s×T=m×0.03×Tcal
Kinetic energy of the bullet
=12mv2=12m(300×102)2ergs
Half of this energy goes into the bullet on impact, therefore
W=12×[12m(300×102)2]=14m×9×108ergsUsingtheformulaW=JH,wehave14m×9×108=(4.2×107)(m×0.03×T)T=9×1084×(4.2×107)×0.03=178.6∘C
:
A
Suppose m be the mass of the bullet and T be the rise in temperature.
Heat produced in the bullet
H=m×s×T=m×0.03×Tcal
Kinetic energy of the bullet
=12mv2=12m(300×102)2ergs
Half of this energy goes into the bullet on impact, therefore
W=12×[12m(300×102)2]=14m×9×108ergsUsingtheformulaW=JH,wehave14m×9×108=(4.2×107)(m×0.03×T)T=9×1084×(4.2×107)×0.03=178.6∘C
Answer: Option A. -> Ice is 68.75gm, and water is 181.25gm at 0∘C.
:
A
The heat, which 0.15kg of water can release when its temperature is changed from 20∘C to 0∘C.
Q1=mWsWΔTW
where, mW is the mass of the water, sW is the specific heat of water and ΔTW is the change in temperature of water.
Given, mW=0.15kg, sW=1kcalkg−1.
Q1=0.15×(1×103)×(20−0)
=3000cal ...(1)
Now, heat absorbed by 0.10kg of ice at −10∘Cto increase its temperature to 0∘C.
Q2=miSiΔTi
where mi is the mass of the ice, si is the specific heat of ice and ΔTi is the change in temperature of ice.
Given, mi=0.10kg, si=0.50kcalkg−1
Q2=0.10×(0.5×103)×[0−(−10)]
=500cal
So, remaining heat,
Q=Q1−Q2=3000−500=2500cal
Now as latent heat of ice is l=80kcalkg−1, the remaining heat will melt ice only
L=Qm⇒m=Qm=250080=31.25g of ice.
Initial amount of ice =0.10kg=100g
So, the remaining ice,
=100−31.25=68.75g
Initial amount of water =0.15kg=150g
Therefore, total water =150+31.25=181.25g
The temperature of the system will be 0∘C.
:
A
The heat, which 0.15kg of water can release when its temperature is changed from 20∘C to 0∘C.
Q1=mWsWΔTW
where, mW is the mass of the water, sW is the specific heat of water and ΔTW is the change in temperature of water.
Given, mW=0.15kg, sW=1kcalkg−1.
Q1=0.15×(1×103)×(20−0)
=3000cal ...(1)
Now, heat absorbed by 0.10kg of ice at −10∘Cto increase its temperature to 0∘C.
Q2=miSiΔTi
where mi is the mass of the ice, si is the specific heat of ice and ΔTi is the change in temperature of ice.
Given, mi=0.10kg, si=0.50kcalkg−1
Q2=0.10×(0.5×103)×[0−(−10)]
=500cal
So, remaining heat,
Q=Q1−Q2=3000−500=2500cal
Now as latent heat of ice is l=80kcalkg−1, the remaining heat will melt ice only
L=Qm⇒m=Qm=250080=31.25g of ice.
Initial amount of ice =0.10kg=100g
So, the remaining ice,
=100−31.25=68.75g
Initial amount of water =0.15kg=150g
Therefore, total water =150+31.25=181.25g
The temperature of the system will be 0∘C.
Answer: Option A. -> 0-1
Answer: (a).0-1
Answer: (a).0-1
Answer: Option C. -> Black body
Answer: (c).Black body
Answer: (c).Black body
Answer: Option D. -> 4 * 10¯⁷ to 1.4 * 10¯⁴ micron meter
Answer: (d).4 * 10¯⁷ to 1.4 * 10¯⁴ micron meter
Answer: (d).4 * 10¯⁷ to 1.4 * 10¯⁴ micron meter
Answer: Option C. -> 0.115
Answer: (c).0.115
Answer: (c).0.115
Answer: Option B. -> False
Answer: (b).False
Answer: (b).False
















