12th Grade > Chemistry
CHEMICAL KINETICS MCQs
Total Questions : 30
| Page 3 of 3 pages
Answer: Option D. -> 1.28×10−2
:
D
From the Arhenius eqn, we know that the rate of a reaction doubles for every 10 K rise in temperature.
k2k1=22⇒k2=3.2×10−3×4=1.28×10−2sec−1
:
D
From the Arhenius eqn, we know that the rate of a reaction doubles for every 10 K rise in temperature.
k2k1=22⇒k2=3.2×10−3×4=1.28×10−2sec−1
Answer: Option D. -> 6.67×10−6mol/l/s
:
D
Rate=Δ[R]Δt=−(0.02−0.03)25×60=6.67×10−6mol/l/s
:
D
Rate=Δ[R]Δt=−(0.02−0.03)25×60=6.67×10−6mol/l/s
Answer: Option D. -> Molecularity of a reaction may be zero or fraction
:
D
Molecularity can never be zero or fractional.
:
D
Molecularity can never be zero or fractional.
Answer: Option D. -> 53.6kJmol−1
:
D
As per Arrhenius equation:
logK2K1=Ea2.3R[T2−T1T2T1]
2.3log2=Ea8.314[10300×310]
∴Ea=53.6kJmol−1
:
D
As per Arrhenius equation:
logK2K1=Ea2.3R[T2−T1T2T1]
2.3log2=Ea8.314[10300×310]
∴Ea=53.6kJmol−1
Answer: Option D. -> 4
:
D
t0.5∝1an−1
Given t0.5∝1a3
Hence, n−1=3
∴n=4
:
D
t0.5∝1an−1
Given t0.5∝1a3
Hence, n−1=3
∴n=4
Answer: Option D. -> rate becomes eight times
:
D
Rate = k[X]3
When X becomes double, rate become 23 = 8 times
:
D
Rate = k[X]3
When X becomes double, rate become 23 = 8 times
Answer: Option D. -> 1
:
D
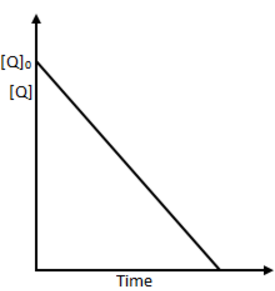
Overall order of reaction can be decided by the data given t75﹪=2t50﹪
∴ It is a first order reaction with respect to P.
From graph [Q] is linearly decreasing with time, i.e., order of reaction w.r.t. Q is zero and the rate expression is r=[P]1[Q]0.
Therefore 1 is the right answer.
:
D
Overall order of reaction can be decided by the data given t75﹪=2t50﹪
∴ It is a first order reaction with respect to P.
From graph [Q] is linearly decreasing with time, i.e., order of reaction w.r.t. Q is zero and the rate expression is r=[P]1[Q]0.
Therefore 1 is the right answer.
Answer: Option A. -> Independent of the initial concentration of the reactant
:
A
Statements in options (b) (c) and (d) correspond to orders 0, 2 and 3 respectively. Hence the answer for the given question is found in the option (a).
:
A
Statements in options (b) (c) and (d) correspond to orders 0, 2 and 3 respectively. Hence the answer for the given question is found in the option (a).
Answer: Option D. -> r=k[A]2
:
D
Larger the order of reaction more the percent increase in the rate of reaction for a given increase in concentration.
:
D
Larger the order of reaction more the percent increase in the rate of reaction for a given increase in concentration.