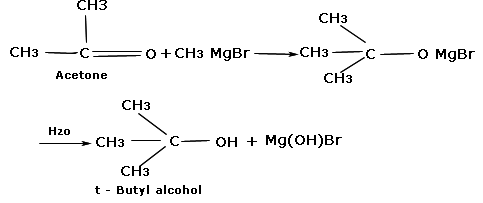
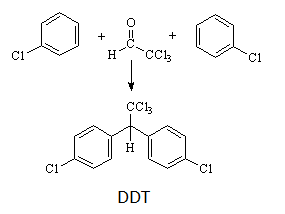
12th Grade > Chemistry
ALDEHYDES AND KETONES MCQs
Total Questions : 30
| Page 2 of 3 pages
Answer: Option B. -> acid chloride
:
B
2CH3COCl+R2Cd→2CH3−CO−R+CdCl2
:
B
2CH3COCl+R2Cd→2CH3−CO−R+CdCl2
Answer: Option C. -> Oppenauer oxidation
:
C
The shown reaction is oxidation of secondary alcohol to ketones using aluminium tert. Butoxide called Oppenauer oxidation.
:
C
The shown reaction is oxidation of secondary alcohol to ketones using aluminium tert. Butoxide called Oppenauer oxidation.
Answer: Option D. -> alkanes are held together by weak van der Waals’ forces (being non-polar), aldehydes and ketones contain polar ╲╱C=O and held together by strong dipole-dipole attraction and lower alcohols have H-bonding, which is stronger than dipole-dipole attraction
:
D
Alcohols have hydrogen bonding and this tendency decreases in the order −OH>−CHO>ketones>R−H
:
D
Alcohols have hydrogen bonding and this tendency decreases in the order −OH>−CHO>ketones>R−H
Answer: Option C. -> C6H5CHO
:
C
C6H5CHO has an aldehyde group and therefore responds to schiff’s test. Due to the absence of α−hydrogens it also gives a positive Cannizzaro’s test but does not reduce Fehling’s solution as the oxidizing agent is not powerful enough to oxidize benzaldehyde to benzoic acid.
:
C
C6H5CHO has an aldehyde group and therefore responds to schiff’s test. Due to the absence of α−hydrogens it also gives a positive Cannizzaro’s test but does not reduce Fehling’s solution as the oxidizing agent is not powerful enough to oxidize benzaldehyde to benzoic acid.
Answer: Option B. -> CHCl2COOH>CH2ClCOOH>CH3COOH
:
B
Greater the number of –I groups on the α-carbon, acidic nature increases .
:
B
Greater the number of –I groups on the α-carbon, acidic nature increases .