Question
We humans have known elemental Sulphur from the earliest times. In the last two decades, the allotropy of sulfur has been explored in great detail. The most common naturally occurring allotrope,S8 cyclo-octasulphur, has a puckered three dimensional “crown” like arrangement of atoms around the ring. This homocyclic allotrope forms “needle-like” crystals above 95∘ C, but below that temperature, crystallizes in a “chunky” fashion. The crystals, which are referred to as monoclinic and rhombic forms, differ simply in the way in which the molecules pack. These are allotropes of each other – True or False?
Answer: Option B
:
B
The question looks long for a true or false type doesn’t it. Shall we keep the answer very short – yes?
Polymorphs are defined as different crystal forms in which identical units of the same compound are packeddifferently. Strictly speaking, the three varieties of cyclo-octasulphur – the α-Sulphur, β-Sulphur and the γ-Sulphur are all polymorphs andnot allotropes. Allotropes contain different molecular units!
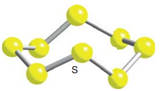
The repeating molecular unit of cyclo-octasulphur – the S8molecule looks like:
Was this answer helpful ?
:
B
The question looks long for a true or false type doesn’t it. Shall we keep the answer very short – yes?
Polymorphs are defined as different crystal forms in which identical units of the same compound are packeddifferently. Strictly speaking, the three varieties of cyclo-octasulphur – the α-Sulphur, β-Sulphur and the γ-Sulphur are all polymorphs andnot allotropes. Allotropes contain different molecular units!
The repeating molecular unit of cyclo-octasulphur – the S8molecule looks like:

Was this answer helpful ?


















Submit Solution