12th Grade > Chemistry
SURFACE CHEMISTRY MCQs
Total Questions : 30
| Page 1 of 3 pages
Answer: Option D. -> AgI ¦ I− ¦ K+
:
D
As excess of KI has been added, I−ions are adsorbed on AgI forming a layer ( and giving it a negative charge). It then attracts the counter ion K+from the medium forming the second layer (diffused layer).
:
D
As excess of KI has been added, I−ions are adsorbed on AgI forming a layer ( and giving it a negative charge). It then attracts the counter ion K+from the medium forming the second layer (diffused layer).
Question 2. Method of formation of solution is given in Column I. Match it with the type of solution given in Column II.
Column I Column II
(i) Sulphur vapours passed through cold water (p) Normal electrolyte solution
(ii) Soap mixed with water above critical micelle concentration (q) Multi molecular colloids
critical micelle concentration
(iii) White of egg whipped with water (r) Associated colloid
(iv) Soap mixed with water below (s) Macro molecular colloids
critical micelle concentration
Column I Column II
(i) Sulphur vapours passed through cold water (p) Normal electrolyte solution
(ii) Soap mixed with water above critical micelle concentration (q) Multi molecular colloids
critical micelle concentration
(iii) White of egg whipped with water (r) Associated colloid
(iv) Soap mixed with water below (s) Macro molecular colloids
critical micelle concentration
Answer: Option A. -> i-q, ii-r, iii-s, iv-p
:
A
Sulphur sol consists of particles containing a thousand or more of S8molecules => Multi molecular colloids
Below CMC, soap with water behaves as a normal electrolyte and above CMC as associated colloid
:
A
Sulphur sol consists of particles containing a thousand or more of S8molecules => Multi molecular colloids
Below CMC, soap with water behaves as a normal electrolyte and above CMC as associated colloid
Answer: Option A. -> Nature of colloid
:
A
This motion is independent of the nature of the colloid but depends on the size of the particles and viscosity of the solution.
:
A
This motion is independent of the nature of the colloid but depends on the size of the particles and viscosity of the solution.
Question 4. Which of the following statements are true about peptization? A. A colloidal sol is converted into precipitate B. It is done in the presence of a small electrolyte C. Charges develop on the precipitate during this process D. The charges developed during this process further breaks up into smaller particles of the size of colloids.
Answer: Option C. -> B,C,D
:
C
During peptization, precipitate is converted into a colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
:
C
During peptization, precipitate is converted into a colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
Question 5. Which of the following statements are false?
A. Freundlich adsorption isotherm holds good for all pressure ranges.
B. The force of attraction existing between the adsorbate and adsorbent are Vander Waal’s forces in case of chemisorption.
C. Adsorption is an endothermic process.
D. The extent of adsorption decreases with increase in temperature for a physiosorption process.
A. Freundlich adsorption isotherm holds good for all pressure ranges.
B. The force of attraction existing between the adsorbate and adsorbent are Vander Waal’s forces in case of chemisorption.
C. Adsorption is an endothermic process.
D. The extent of adsorption decreases with increase in temperature for a physiosorption process.
Answer: Option B. -> A,B,C
:
B
A. Freundlich isotherm doesn't hold good at high pressures as it seems to attain saturation at high pressure.
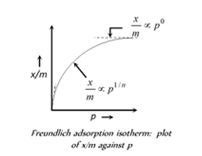
B. Vander Waal's forces exit in case of physiosorption; in chemisorption chemical bonds are formed.
C. Adsorption is an exothermic process, as adsorption process is followed by release of heat.
D. This statement is true. Since adsorption is an exothermic process, so Le chatelier's principle explains this property.
:
B
A. Freundlich isotherm doesn't hold good at high pressures as it seems to attain saturation at high pressure.

B. Vander Waal's forces exit in case of physiosorption; in chemisorption chemical bonds are formed.
C. Adsorption is an exothermic process, as adsorption process is followed by release of heat.
D. This statement is true. Since adsorption is an exothermic process, so Le chatelier's principle explains this property.
Answer: Option C. -> The amount of the electrolyte in millimoles required to bring about the coagulation of 1 litre of colloidal sol
:
C
By definition
:
C
By definition
Answer: Option B. -> A,B,D
:
B
A.It is irreversible in nature because of the formation of new bonds.
B.Surface area of a powdered solid adsorbent depends on its particle size. Smaller the particle size, greater is its surface area and hence more is the extent of adsorption.
C.Correct statement
D.In case of chemisorption isobar, it first increases and then decreases with increase in temperature.
:
B
A.It is irreversible in nature because of the formation of new bonds.
B.Surface area of a powdered solid adsorbent depends on its particle size. Smaller the particle size, greater is its surface area and hence more is the extent of adsorption.
C.Correct statement
D.In case of chemisorption isobar, it first increases and then decreases with increase in temperature.
Answer: Option D. -> d
:
D
The equation is: xm=kp1n⇒ln(xm)=lnk+(1n)lnp⇒lnx−lnmk+(1n)lnp
lnx=lnm+lnk+(1n)lnp⇒lnx=lnmk+(1n)lnp
:
D
The equation is: xm=kp1n⇒ln(xm)=lnk+(1n)lnp⇒lnx−lnmk+(1n)lnp
lnx=lnm+lnk+(1n)lnp⇒lnx=lnmk+(1n)lnp
Answer: Option B. -> Micelle formation by soap in aqueous solution occurs above a particular concentration.
:
B
The formation of micelles takes place only above a particular temperature called ‘Krafft Temperature’ and only above a particular concentration called ‘Critical micelle concentration’.
On dilution of soap solution micelles revert to individual ions.
:
B
The formation of micelles takes place only above a particular temperature called ‘Krafft Temperature’ and only above a particular concentration called ‘Critical micelle concentration’.
On dilution of soap solution micelles revert to individual ions.
Answer: Option B. -> Heterogeneous catalysis
:
B
Here the reactant is in gaseous phase while the catalyst is in solid phase. Since the two are in different phases, so this is an example of heterogeneous catalysis.
:
B
Here the reactant is in gaseous phase while the catalyst is in solid phase. Since the two are in different phases, so this is an example of heterogeneous catalysis.
















