MCQs
Total Questions : 151
| Page 5 of 16 pages
Answer: Option C. -> Its output potential is dependent on the composition of the solution
Answer: (c).Its output potential is dependent on the composition of the solution
Answer: (c).Its output potential is dependent on the composition of the solution
Answer: Option D. -> Its output potential is zero volts
Answer: (d).Its output potential is zero volts
Answer: (d).Its output potential is zero volts
Answer: Option C. -> Hilderbant Hydrogen electrode
Answer: (c).Hilderbant Hydrogen electrode
Answer: (c).Hilderbant Hydrogen electrode
Answer: Option B. -> 1
Answer: (b).1
Answer: (b).1
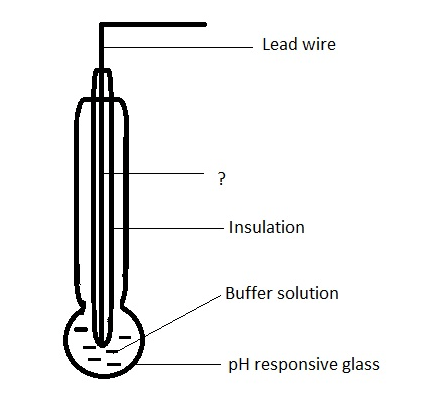
Answer: Option C. -> It gives a salt error
Answer: (c).It gives a salt error
Answer: (c).It gives a salt error
Answer: Option A. -> True
Answer: (a).True
Answer: (a).True
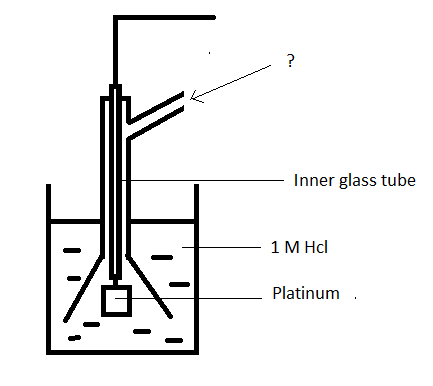
Answer: Option A. -> Hydrogen at 1 atm
Answer: (a).Hydrogen at 1 atm
Answer: (a).Hydrogen at 1 atm
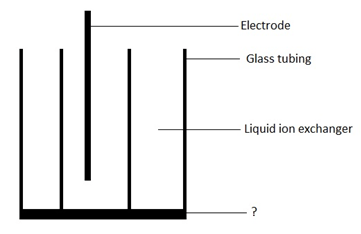
Answer: Option C. -> Hydrophobic material
Answer: (c).Hydrophobic material
Answer: (c).Hydrophobic material
Answer: Option B. -> Silver wire coated with silver chloride
Answer: (b).Silver wire coated with silver chloride
Answer: (b).Silver wire coated with silver chloride
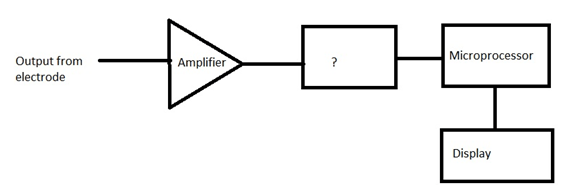
Answer: Option C. -> A/D converter
Answer: (c).A/D converter
Answer: (c).A/D converter