12th Grade > Chemistry
P-BLOCK GROUP 18 - NOBLE GASES MCQs
Total Questions : 12
| Page 1 of 2 pages
Answer: Option A. -> True
:
A
All these noble gases are monoatomic gases at room temperature. The atomicity is explained by the stable valence electron configuration. Can you recall what the valence configuration in general is?
These elements are gases at room temperature because in the liquid/solid states, very weak dispersion forces hold these atoms together. So to reach such states, large negative Celsius temperatures are required. Let us look at the values:
NoblegasMeltingpoint(∘C)Boilingpoint(∘)CNumberofelectronsHe−−2692Ne−249−24510Ar−189−18618Kr−157−15236Xe−112−10954Rn−71−6286
As you can see, the largest range between melting point and boiling point is for Rn and this is <10 degrees Celsius. Hence the statement in the question is true!
:
A
All these noble gases are monoatomic gases at room temperature. The atomicity is explained by the stable valence electron configuration. Can you recall what the valence configuration in general is?
These elements are gases at room temperature because in the liquid/solid states, very weak dispersion forces hold these atoms together. So to reach such states, large negative Celsius temperatures are required. Let us look at the values:
NoblegasMeltingpoint(∘C)Boilingpoint(∘)CNumberofelectronsHe−−2692Ne−249−24510Ar−189−18618Kr−157−15236Xe−112−10954Rn−71−6286
As you can see, the largest range between melting point and boiling point is for Rn and this is <10 degrees Celsius. Hence the statement in the question is true!
Answer: Option B. -> Helium atoms are small and cannot be retained by the Earth's gravitational field.
:
B
Helium accounts for up to 23 percent by mass of the Universe and the Sun. Itis the most abundant element in the universe after hydrogen; Helium is rare in the atmosphere because its atoms are small and travel fast enough to escape the pull of Earth’s gravitational field. Do note that all the other noble gases occur in the atmosphere. There is no primordial Helium. In other words, there wasno Helium from before the formation of Earth.
That leaves us with the question – where does the Helium on Earth come from? Helium comes into existence as α particles from radioactive decay and tends to accumulate in natural gas deposits.
:
B
Helium accounts for up to 23 percent by mass of the Universe and the Sun. Itis the most abundant element in the universe after hydrogen; Helium is rare in the atmosphere because its atoms are small and travel fast enough to escape the pull of Earth’s gravitational field. Do note that all the other noble gases occur in the atmosphere. There is no primordial Helium. In other words, there wasno Helium from before the formation of Earth.
That leaves us with the question – where does the Helium on Earth come from? Helium comes into existence as α particles from radioactive decay and tends to accumulate in natural gas deposits.
:
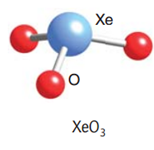
Let us draw the Lewis structure for Xenon (VI) oxide or Xenon trioxide:
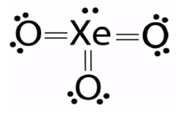
As we can see from the Lewis structure, there is one lone pair on the central atom and there are three atoms attached to the Xenon atom. Using VSEPR theory for a steric number = 3 bp + 1 lp = 4, we get a tetrahedral geometry and a pyramidal shape.
Answer: Option D. -> No reaction
:
D
For the reaction,
Xe(g)+O2(g)→xenon oxidesΔfG∘ > 0
In other words, the standard change in free energy of the reaction is positive. Hence, this reaction is non-spontaneous. None of the Xenon oxides can be synthesized from this approach. So how can they be obtained?
:
D
For the reaction,
Xe(g)+O2(g)→xenon oxidesΔfG∘ > 0
In other words, the standard change in free energy of the reaction is positive. Hence, this reaction is non-spontaneous. None of the Xenon oxides can be synthesized from this approach. So how can they be obtained?
Answer: Option E. -> None of the above
:
E
All these noble gases are nearly inert (except Xenon and to a small extent, Krypton). Definitely they can be used to create inert atmospheres – particularly Argon. Helium is much lighter than air and it is used to keep airships and air balloons afloat in the air. Owing to abundance, Argon is manufactured on a much larger scale than the other noble gases. It finds extensive application in high-temperature metallurgical processes as an inert atmosphere.
Helium is extremely difficult to solidify even at 1 K. At this temperature, a pressure of 2.5 MPa is needed to solidify the liquid Helium! This property is utilized to cool superconducting magnets which become superconducting at very low temperatures. Liquid Helium is a subject of tremendous interest in the scientific community. At 100 kPa, the gas liquefies at 4.2 K. Let us call this Helium-I. When this liquid is cooled further to about 2.2 K, the properties of the liquid change dramatically. Let us call this Helium-II. Its viscosity drops to 0!
Helium-II conducts heat 106times better as compared to the thermal conductivity of Helium-I.
In what is popularly referred to as “neon signs”, using different gases like He, Ne, Ar, Kr & Xe inside the tubes produce characteristic colours depending on the gas used. For example, neon gives red light and helium yellow light. Xenon flash lamps produce short bursts of UV and visible
light. Further, these noble gases are also used in lasers (helium–neon, argon-ion, and krypton-ion lasers).
As we can see, all the uses are appropriate. Hence,
e) none of the above
:
E
All these noble gases are nearly inert (except Xenon and to a small extent, Krypton). Definitely they can be used to create inert atmospheres – particularly Argon. Helium is much lighter than air and it is used to keep airships and air balloons afloat in the air. Owing to abundance, Argon is manufactured on a much larger scale than the other noble gases. It finds extensive application in high-temperature metallurgical processes as an inert atmosphere.
Helium is extremely difficult to solidify even at 1 K. At this temperature, a pressure of 2.5 MPa is needed to solidify the liquid Helium! This property is utilized to cool superconducting magnets which become superconducting at very low temperatures. Liquid Helium is a subject of tremendous interest in the scientific community. At 100 kPa, the gas liquefies at 4.2 K. Let us call this Helium-I. When this liquid is cooled further to about 2.2 K, the properties of the liquid change dramatically. Let us call this Helium-II. Its viscosity drops to 0!
Helium-II conducts heat 106times better as compared to the thermal conductivity of Helium-I.
In what is popularly referred to as “neon signs”, using different gases like He, Ne, Ar, Kr & Xe inside the tubes produce characteristic colours depending on the gas used. For example, neon gives red light and helium yellow light. Xenon flash lamps produce short bursts of UV and visible
light. Further, these noble gases are also used in lasers (helium–neon, argon-ion, and krypton-ion lasers).
As we can see, all the uses are appropriate. Hence,
e) none of the above
:
Xenon forms three fluorides:
Xe(g)+F2(g)400∘C1atm−−−−−−−→XeF2(s) {Xe in excess}
Xe(g)+2F2(g)600∘C6atm−−−−−−−→XeF4(s){Xe:F2=1:1.5}
Xe(g)+3F2(g)300∘C50atm−−−−−−−−→XeF6(s){Xe:F2=1:20}
Answer: Option A. -> True
:
A
True.
2XeF2(s)+2H2O(l)→2Xe(g)+O2(g)+4HF(l)
6XeF4(s)+12H2O(l)→4Xe(g)+3O2(g)+2XeO3(s)+24HF(l)
XeF6(s)+3H2O(l)→XeO3(s)+6HF(l)
Hydrolysis of XeF6 is a multi-step process. Initially Xenon oxide tetrafluoride – XeF4 is formed.
XeF6(s)+H2O(l)→XeOF4(l)+2HF(l)
Followed by
XeOF4(l)+2H2O(l)→XeO3(s)+4HF(l)
:
A
True.
2XeF2(s)+2H2O(l)→2Xe(g)+O2(g)+4HF(l)
6XeF4(s)+12H2O(l)→4Xe(g)+3O2(g)+2XeO3(s)+24HF(l)
XeF6(s)+3H2O(l)→XeO3(s)+6HF(l)
Hydrolysis of XeF6 is a multi-step process. Initially Xenon oxide tetrafluoride – XeF4 is formed.
XeF6(s)+H2O(l)→XeOF4(l)+2HF(l)
Followed by
XeOF4(l)+2H2O(l)→XeO3(s)+4HF(l)
Answer: Option B. -> False
:
B
Xenon Fluorides are clean fluorinating agents because usually they yield the fluorinated product and Xe gas. The required product can then easily be separated from Xe gas. In the following reaction,
XeF2(s)+H2C=CH2(g)→Xe(g)+H2CF−FCH2(g)
XeF2 is reduced to Xe (g). Definitely Xenon fluorides are not reducing agents. On the contrary, they behave as oxidizing agents. For example,
XeF4(s)+2SF4(g)→Xe(g)+2SF6(g)
:
B
Xenon Fluorides are clean fluorinating agents because usually they yield the fluorinated product and Xe gas. The required product can then easily be separated from Xe gas. In the following reaction,
XeF2(s)+H2C=CH2(g)→Xe(g)+H2CF−FCH2(g)
XeF2 is reduced to Xe (g). Definitely Xenon fluorides are not reducing agents. On the contrary, they behave as oxidizing agents. For example,
XeF4(s)+2SF4(g)→Xe(g)+2SF6(g)
Answer: Option A. -> True
:
A
This is true.
Xenon is the only noble gas to form a variety of compounds and then only with highly electronegative elements, such as fluorine, oxygen, and to some extent - nitrogen.
With oxygen, Xenon forms two oxides - XeO3andXeO4
It forms three fluorides with fluorine. What are their formulae?
Interestingly, Xenon also forms two oxyfluorides : XeO2F2andXeOF4
:
A
This is true.
Xenon is the only noble gas to form a variety of compounds and then only with highly electronegative elements, such as fluorine, oxygen, and to some extent - nitrogen.
With oxygen, Xenon forms two oxides - XeO3andXeO4
It forms three fluorides with fluorine. What are their formulae?
Interestingly, Xenon also forms two oxyfluorides : XeO2F2andXeOF4
Answer: Option A. -> Square planar and octahedral
:
A
Let us look at the ground state and the second excited state configurations: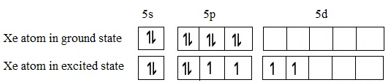
These 5 orbitals may be assumed to hybridize to correspond to sp3d2 geometry. The 4 bond pairs (covalent bonds between Xe central atom and F atoms) lie in a plane symmetrically while the two lone pairs are distributed symmetrically above and below this plane.
Geometry is square bipyramidal or octahedral. For shape, we do not take the lone pairs into account. So shape will only be square planar.
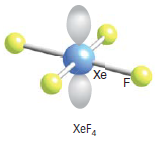
What would be the shape of XeF6? I will give you the answer but I encourage you to arrive at it by reason. You can assume that it has 6 bonding pairs and one lone pair:
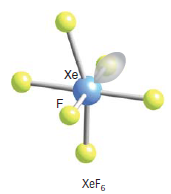
Experimental evidence suggests that the structures of XeF2andXeF4 are exactly as those predicted by VSEPR theory. For XeF6, given that the steric number = 6 bp + 1lp = 7, we can be tempted to conclude that it is similar to Iodine heptafluoride, thus having a pentagonal bipyramidal structure. But it has a face-capped octahedral structure. It is a structure where one lone pair is facing through one of the faces of the octahedron. In terms of the Valence bond theory, we could reason three of the ground state 5p electrons be promoted to the 5d orbitals giving the possibility of an sp3d3 hybridization. Molecular orbital theory fails to explain the actual structure of XeF6. According to MOT, the structure should be a completely symmetrical octahedral one. However, this is not the case. In fact, the structure of the molecule is “fluxional”. What do we mean by that? It simply means that the structure is constantly fluctuating between structures where the lone pair spreads across each of the 8 triangular faces.
:
A
Let us look at the ground state and the second excited state configurations:

These 5 orbitals may be assumed to hybridize to correspond to sp3d2 geometry. The 4 bond pairs (covalent bonds between Xe central atom and F atoms) lie in a plane symmetrically while the two lone pairs are distributed symmetrically above and below this plane.
Geometry is square bipyramidal or octahedral. For shape, we do not take the lone pairs into account. So shape will only be square planar.

What would be the shape of XeF6? I will give you the answer but I encourage you to arrive at it by reason. You can assume that it has 6 bonding pairs and one lone pair:

Experimental evidence suggests that the structures of XeF2andXeF4 are exactly as those predicted by VSEPR theory. For XeF6, given that the steric number = 6 bp + 1lp = 7, we can be tempted to conclude that it is similar to Iodine heptafluoride, thus having a pentagonal bipyramidal structure. But it has a face-capped octahedral structure. It is a structure where one lone pair is facing through one of the faces of the octahedron. In terms of the Valence bond theory, we could reason three of the ground state 5p electrons be promoted to the 5d orbitals giving the possibility of an sp3d3 hybridization. Molecular orbital theory fails to explain the actual structure of XeF6. According to MOT, the structure should be a completely symmetrical octahedral one. However, this is not the case. In fact, the structure of the molecule is “fluxional”. What do we mean by that? It simply means that the structure is constantly fluctuating between structures where the lone pair spreads across each of the 8 triangular faces.
















